Clustering and Targeting Stakeholder Networks
Applying graph theory to analyze and engage the complex network of stakeholders who influence the successful launch of a new product.

In a previous post, I introduced the concept of Temporal Multi-Party Analysis (TEMPA) as a model for understanding the time-based behaviour of complex connected systems and entities.
A wide variety of state-of-the-art research is currently being done in the field of medicine with graph-based knowledge representation. Since graphs are the most universal and natural language for modeling data, entities and systems with complex connections, they are an intuitive choice for this purpose.
For research, development and biological studies, graphs provide a natural representation as a network of cells and molecules that interact with each other. This biological network representation has been a powerful resource for understanding the properties of biological systems, from single cell to population level. Emergent research that applies graph neural networks to these biological networks has been very successful in driving some remarkable discoveries, including drug discovery, protein function prediction, disease diagnosis and precision medicine.
The Stanford Network Analysis Project has been developing advanced machine learning models for networks in biology and medicine. The work of this group encompasses Graph Neural Networks (GNNs) for studying protein-protein interactions, disease pathways, gene function predictions and deep learning on hierarchical multi-relational graphs to study human tissue, cellular structures, drug interactions and side effects.
Additionally, graph representational learning is also being applied to patient Electronic Health Records (EHRs) to learn how medical entities that are represented in EHR data, including diagnoses, prescriptions, medical procedures, doctor profiles, and patient demographics, etc. connect together. A study of patient similarity in these graph representations is meant to enable novel healthcare applications such as predictive diagnosis, cohort analysis and personalized medicine.
The application of graph-based analytics and deep machine learning is certainly enabling novel techniques and algorithms for scientific discovery and understanding in the field of medicine.
However, in addition to biological applications at the microscopic scale, there is also a significant opportunity to apply graph-based techniques at the macroscopic scale.
This is where Temporal Multi-Party Analysis (TEMPA) models can be applied to study macro-level systems and dynamics that evolve over time.
Biopharmaceutical Product Launches: A Complex Multi-Party Affair
The pharmaceutical industry underwent a significant patent expiration event, known as the ‘patent cliff’, between the years 2010–2016. During this time period, a vast majority of brand name drugs that accounted for large percentages of big pharma company sales, lost patent protection. The patent cliff drove a major shift in generic drugs manufacturing to locations like India and China. Some of the implications of this move from a drug shortage perspective have been analyzed in a separate post.
A secondary change that arose from the patent cliff was a pivot across all major pharmaceutical manufacturers to a biotech model where the R&D investment shifted into more complex and specialty biotechnology-based medicines which provide a higher barrier to entry for generics manufacturers. Also, advances in genomics research and the availability of cheaper computational resources for genetic data has further led to widespread market proliferation of smaller specialty biotech startups.
These market trends have led the way for inclusion of highly specialized scientific and medical knowledge into the go-to-market models for most Bio-Pharma therapies. This has further created an increasing need to drive targeted digital content and product value discussions for all stakeholders that are involved in the decision-making process. In response, Bio-Pharma companies are compiling more and more evidence data and scientific insights.
However, many companies have difficulty communicating the science and value proposition effectively with the growing array of stakeholders that influence purchasing decisions. It is therefore key to understand how medical decision makers consume scientific information and form opinions of product value and effectiveness. This will enable both commercial operations and medical affairs functions at Bio-Pharma companies to generate and present high-quality scientific knowledge to the market and educate stakeholders about next-generation products.
Given the changing nature of digital habits and demographics of medical decision makers, the preferred channels and sources of medical information are also shifting to digital channels and professional networks that bring with them credibility in the medical and professional communities. In order to control the scientific and clinical value narrative for their products, the Bio-Pharma industry needs to focus on influential stakeholders and opinion leaders who ‘tip the scale’ for discussions in these communities and clusters.
Engagement relies on the ability to first effectively identify and understand the information needs of clusters in these stakeholder networks and then generate tailored targeted content for the cluster. In a clustered market that includes various categories of medical stakeholders, content deployment strategies need to target each cluster based on the cluster’s priorities regarding product attributes that influence their decision-making and content consumption preferences.
Therapy selection and adoption decisions are influenced by five broad categories of stakeholders that may prioritize different criteria for measuring the ‘value’ and ‘performance’ of a specialty drug product.
Healthcare professionals: Physicians, Nurses and other prescribers
Patients: Individuals and Patient Advocacy Groups
Institutional decision-makers: Leaders at Payer Institutions, Pharmacy Chains, Integrated Care Networks, Group Purchasing Organizations, Hospitals, Healthcare Systems, etc.
Medical Organizations: Clinical Practice Guideline & Treatment Pathway Developers, Medical Associations, Medical Professional Networks, etc.
Regulators: Regulatory agencies, pricing and market authorization related organizations
Given the social and professional connections of these different stakeholders, the dynamics of their network plays a vital role in determining the structure of clusters that may show strong correlation in both information needs and decision-making criteria.
Stakeholders that bridge and link different clusters or stakeholder types can also be instrumental in driving cross-cluster influence, for instance medical experts that have close ties to key reimbursement decision makers. Stakeholder bridges also provide communication pathways and message penetration to institutional stakeholders that may be otherwise hard to reach.
We can build a Temporal Multi-Party Analysis (TEMPA) model to understand and visualize the structure and dynamics of these stakeholder clusters over time. The characteristics of a network topology of stakeholders can be analyzed by the following methods:
Clustering: identifying densely linked communities or clusters of nodes with modularity
Eigencentrality: identifying nodes with high degree of centrality or influence
Link prediction: predicting the strength or presence of links between nodes with triadic closures, articulation points and bridges
Similarity: identifying clusters that have similar structures
In a clustered market, applying community detection and similarity methods will drive efficiencies in designing the scientific and marketing content that targets information needs of each cluster. By targeting influential nodes within clusters, there are strong synergies in delivering product value and product performance messages as well as in identifying barriers to adoption.
Based on centrality measures, individual stakeholders take on different levels of importance, so central stakeholders are apt to be much more impactful and influential in driving opinion than isolated experts.
Depending on context, stakeholders in each of the clusters may prioritize different product attributes that contribute to their opinions and decision-making regarding the adoption of a particular therapy.
The product attributes include both clinical and non-clinical properties: pharmacodynamics, toxicology, dosing regimen, therapy administration, clinical efficacy, clinical safety profile, real-world evidence (RWE), patient reported outcome measures (PROMs), patient support programs (PSPs), side effects, adverse events, breadth of manufacturer’s portfolio, brand reputation, value economics, pricing, etc.
For stakeholder clusters most focused on understanding product value and controlling costs, Bio-Pharma companies must focus on new ways to leverage existing data and content to make compelling value arguments. Other stakeholder clusters such as patient groups and nursing staff may instead prioritize ease of therapy administration, side effects and availability of support programs to manage adverse events. Meanwhile, Practice Managers at oncology clinics may prioritize the availability and accessibility of reimbursement programs for effectively engaging with insurance companies on behalf of patients.
Using a Temporal Multi-Party Analysis (TEMPA) model to identify stakeholder clusters and their specific priorities provides a framework for the assembly of targeted content that speaks directly to the information needs of each cluster.
Knowledge of stakeholder position in the network topology and their connections also provides a signal for additional product attribute data that should be included in the content assembly for specific influential stakeholders because of their reach within the network.
Once the structure of the stakeholder network has been analyzed and target clusters identified, content can be assembled for each cluster based on their communication priorities. Delivery strategies for content per cluster is implemented in compliant coordination between the Bio-Pharma’s Commercial, Medical Affairs, Advocacy and Public Relations teams over digital and in-person channels.
The example below models a hypothetical network topology of stakeholders in the regional market of a specialty drug product ONC123 for lung cancer, including the following stakeholders:
O1…Oa — oncologists that specialize in lung cancer treatment.
N1…Nb — nurse practitioners responsible for administering the treatment regimen.
P1…Pc and PAG — patients currently undergoing lung cancer treatment and the national patient advocacy group for lung cancer patients.
H1…Hd — hospitals that provide lung cancer treatment.
PT1…PTd — P&T committees at the hospitals H1…Hd.
RM1…RMd — Reimbursement managers at hospitals H1…Hd.
C1…Ce — oncology clinics hospitals that provide lung cancer treatment.
PM1…PMe — Practice managers at oncology clinics C1…Ce.
IDN — provider network / integrated delivery network that provides lung cancer treatment
PH1…PHf — Pharmacy directors or managers at specialty pharmacies that dispense ONC123.
IP1…IPg — Decision Makers/Leaders at payers and insurance providers.
CCN — Cancer care network, a national body that develops treatment guidelines and pathways.
SMO — Society for medical oncology that holds national and regional congresses/conferences.
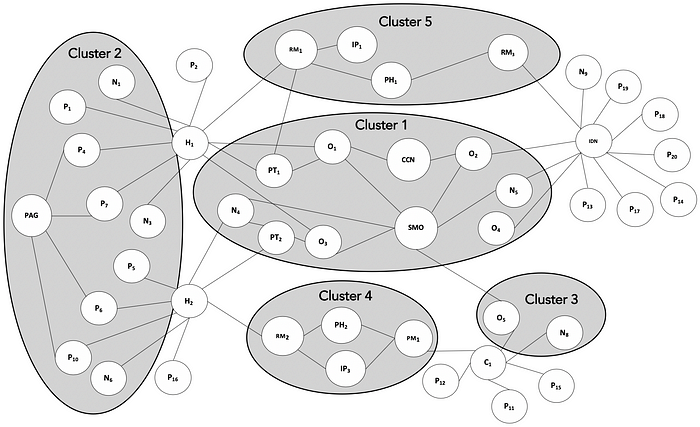
Cluster 1 is made up of influential oncologists, nurse practitioners, IDN and P&T members who have a major say in guideline and pathway development and are key opinion leaders at major medical associations as well as large healthcare institutions. This cluster will be most interested in population-level aspects of the disease, health economics, outcomes research and the key mechanisms underpinning the development of the disease and therapeutic approaches.
Cluster 2 is made up of caregivers, patient community, advocacy groups and frontline nursing staff. This cluster will be most interested in topics such as educational resources, key outcome statistics, treatment regimen, dosing, administration, treatment adherence, patient support programs, side-effect and adverse event management.
Cluster 3 is made up of medical oncologists and care providers who see patients at smaller clinics. This cluster will be most interested in the latest information about the disease, available treatment options and the tools to educate patients and answer their questions.
Cluster 4 is made up of hospital administrators, pharmacy managers and clinic practice managers who assist patients with dispensing and reimbursement. This cluster will be most interested in pricing, reimbursement mechanisms, rebates and financial support programs.
Cluster 5 is made up of leaders at payer institutions along with influential administrators from large healthcare providers and integrated delivery networks. This cluster will be most interested in information about disease epidemiology, relative clinical and health economic impact of disease progression like hospitalization rates and data/analyses on product value.
The information needs and engagement strategy for each cluster will vary over time through multiple steps that are involved in product launch, therapy adoption and procurement decisions, including the following — developing disease understanding, diagnosis, treatment selection, treatment administration, reimbursement, patient monitoring, follow-up and data gathering for real world evidence.
Application of TEMPA for cluster targeting and content delivery
A Temporal Multi-Party Analysis (TEMPA) model can be used by the Bio-Pharma manufacturer of ONC123 to understand the information needs and priorities of each stakeholder cluster with respect to their therapy, and then develop engagement strategies and content specific to those needs.
For the TEMPA model, let’s assume Î = {1,2,3, … ,T} is a set of discrete time intervals over the lifecycle of drug product ONC123.
P (t)(k) is the set of weights that measure the relative importance of product attributes that influence the adoption decision of stakeholder node cluster k at time t. The weights are derived from multiple data sources such as Customer Relationship Management (CRM) systems, stakeholder interactions, correspondence, online posts, articles, medical publications, industry research and other digital data exhausts. When data is available at the node level, the cluster weight is the sum of node weights.
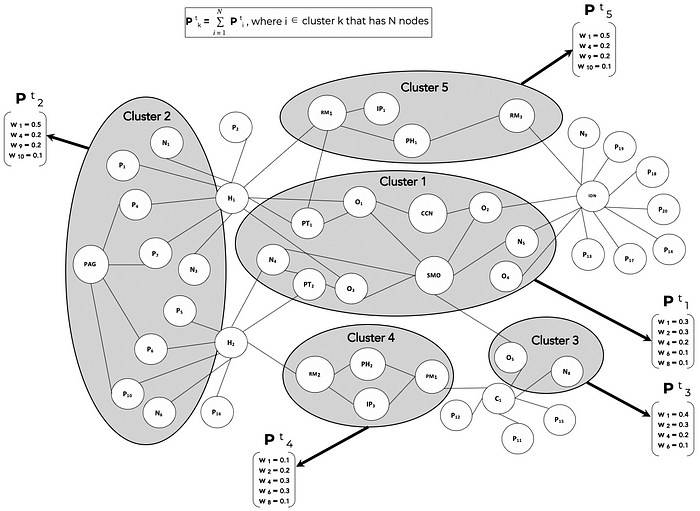
Over the lifecycle of ONC123, from clinical trials through pre-launch and then post-launch, the stakeholder clusters will evolve in time and so will the content needs of each cluster. TEMPA is used to model these dynamics both in terms of the cluster compositions as well as the information needs per cluster.
C (t)(k) is the content that is delivered to stakeholder cluster k at time t. The content piece C (t)(k) is assembled from the content library by using the relative importance weights of attributes in P (t)(k). The content library contains all the data assets (manufacturing process, stability studies, non-clinical data, clinical studies, clinical trial data, co-morbidity studies, efficacy and outcomes data, disease information, patient education material, value economic studies, etc.) that are gathered and catalogued during the drug development, manufacturing, clinical trial, regulatory approval, product launch and commercialization process.
As depicted in Figure 3 below, once the communication priorities of each cluster have been identified for a time interval t and have also been quantified as relative weights of attributes and data points, the targeted content for each cluster can be auto-assembled from the library of raw data assets that have been created over the course of product development and commercialization.
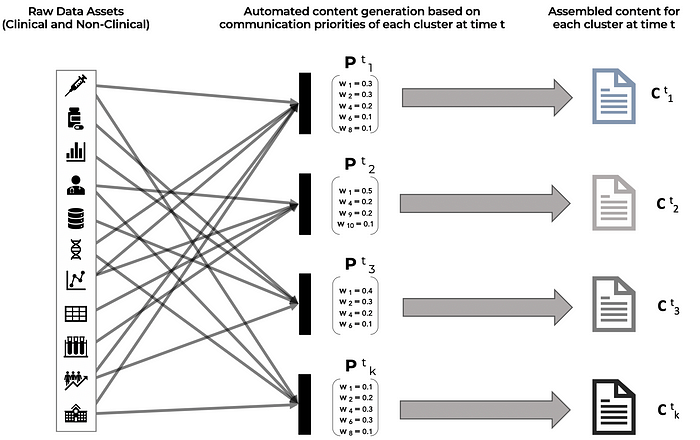
As an additional nuance, if psycholinguistic information is available through the analysis of stakeholder emails, messages, social posts, online commentary, etc. then the tonality and choice of words in the content pieces for each cluster can also be adjusted for alignment with the psychographic profiles of the target audience, ensuring that the key messages are received and processed as intended for the stakeholders in the cluster.
S (t)(i) is the engagement state (represented as binary value of 0 or 1) of node i in the cluster k at time t.
The binary value of S (t)(i) is set 0 or 1 based on engagement of the stakeholder audience with the targeted content C (t)(k) that is delivered to the cluster. This data comes from omni-channel engagement platforms used by the company. For a cluster k with N nodes, the overall cluster engagement state is the relative proportion of engaged nodes.
This is illustrated below in Figure 4 as different content pieces C (t)(k) delivered to different clusters, individual node engagement as dark nodes that have S (t)(i) = 1, and the aggregate engagement per cluster.
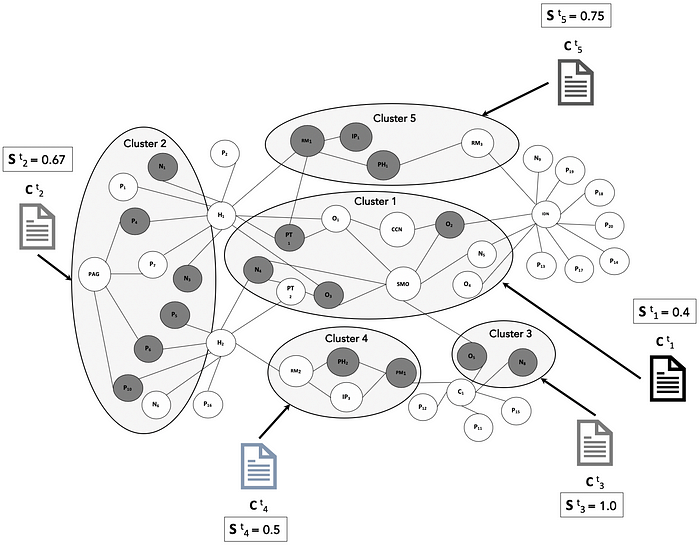
Once the content is assembled for each cluster, the delivery to stakeholders within each cluster follows the appropriate alignment, coordination and compliance firewalling between commercial operations and medical affairs functions. As the content is delivered through digital and in-person channels, engagement data is recorded to measure the effectiveness of the content in meeting the stakeholder’s information needs.
Conclusion
As illustrated, the use of TEMPA model allows the Bio-Pharma manufacturer to develop a deep understanding of the information needs and decision criteria of each stakeholder cluster. This understanding is temporal and must account for how the clusters will evolve and how the needs and expectations of different stakeholder clusters change over time through the product’s lifecycle.
Once stakeholder needs are understood for each time period time t and content assembly completed in alignment with stakeholder interests, the delivery strategies should include appropriate synchronization between digital channels that engage stakeholders with cluster-specific communication and individual follow-ups and in-person visits.
While the example provided here is for engaging stakeholder clusters and generating targeted content for medical stakeholders in a specialty drug launch, the TEMPA model can obviously be extended for customer cluster analysis, engagement strategies and targeted content development for any other product or service industry.
References
1. D. Watts (2002) A simple model of global cascades on random networks
2. L. Plantevin et al. (2017) Reinventing the role of medical affairs
3. J. Eaves (2019) Blog Series on Oncology Product Commercialization
4. T. Wu et al. (2019) Representation Learning of EHR Data via Graph-Based Medical Entity Embedding
5. W. L. Hamilton et al. (2018) Representation Learning on Graphs: Methods and Applications